
Microscopy-guided protein isolation and identification at the level of individual organelles is increasingly crucial across numerous biological fields. Fields such as pathology, neuroscience, oncology, and cell biology rely on microscopy techniques to detect, localize, and map the critical features within cells. However, it has historically been a significant challenge to isolate and identify proteins within specific subcellular regions that are relevant to pathological signatures or crucial molecular interactions. To address this need, the field of "Scoop Biology" emerged to bridge the gap between scientific inquiry and technological breakthroughs to thoroughly investigate and comprehend spatial biological dynamics across various disciplines.
What is Scoop Biology?
"Scoop" here is synonymous with the precise isolation or extraction of specific biological samples for downstream proteomic analysis. Scoop Biology entails the development and implementation of sophisticated methodologies and instruments designed for the meticulous isolation of regions of interest (ROIs) within biological specimens. These ROIs at a subcellular precision often signify the hallmarks of a disease or highlight pivotal molecular interaction and these new techniques enable de novo spatial proteomics discovery and shed light on the intricate landscape of protein distribution within cells and tissues. Additionally, Scoop Biology also encompasses the research and formulation of data processing algorithms and user-centric software solutions, all designed to enhance the precision and clarity of spatial biological data interpretation.
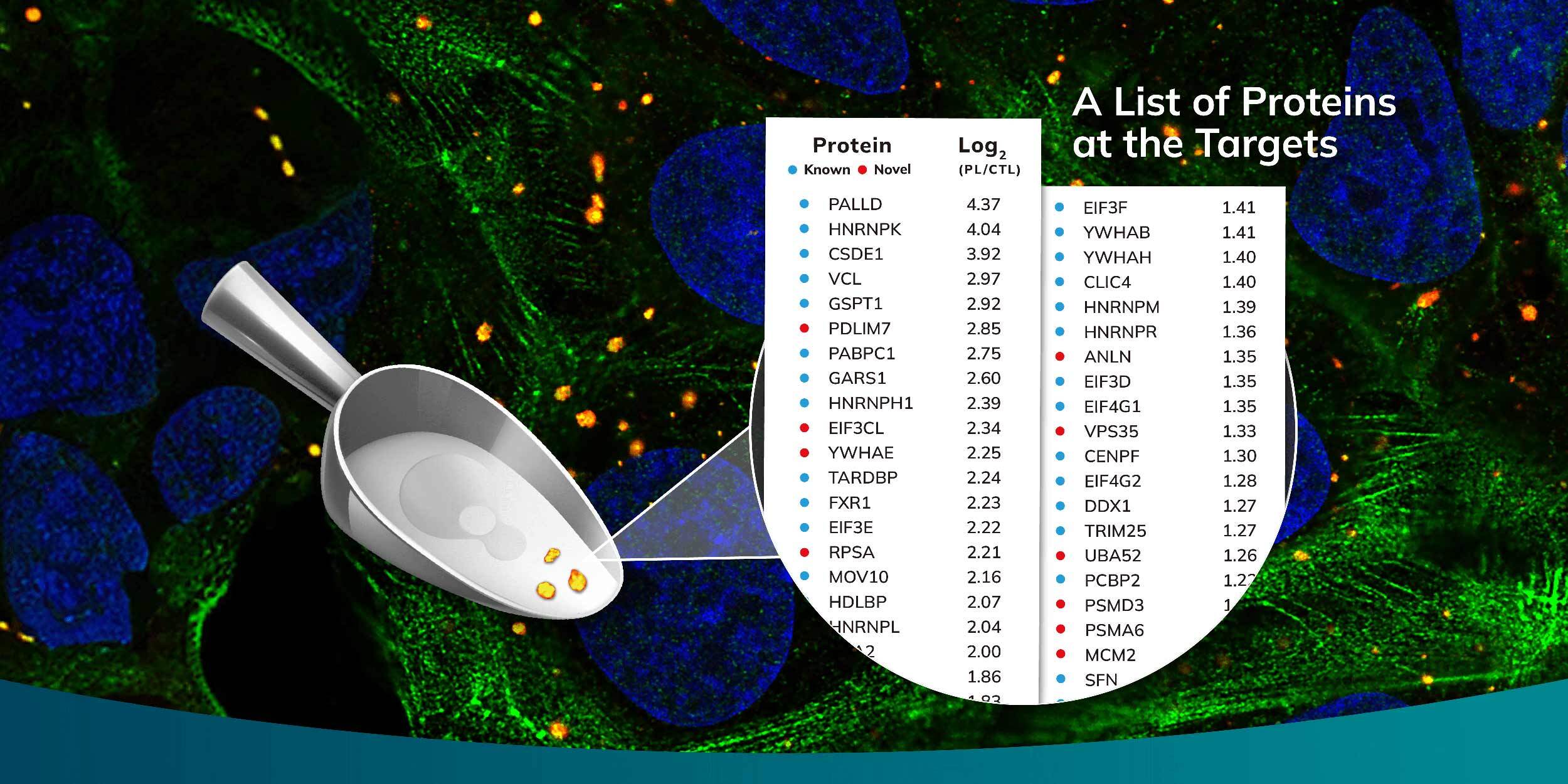
The capability to selectively isolate proteins via microscopic observation from a vast population of cells, accompanied by the application of mass spectrometry for detailed protein identification, has paved the way for unprecedented discoveries in subcellular proteomics. At Syncell, we have harnessed the potential of Scoop Biology through our innovative Microscoop™ system, a platform that enables precision in situ subcellular photo-biotinylation of proteins. This process is tailored to user-specified ROIs, methodically addressing one field of view (FOV) after the next, and can be applied to thousands of FOVs fully autonomously.
Our Microscoop system adeptly calculates the required illumination pattern for biotinylation by leveraging image processing techniques and artificial intelligence (AI), optimizing the targeting process across analogous ROIs. Once a substantial quantity of proteins has been biotinylated in either a fixed cell or formalin-fixed paraffin-embedded (FFPE) tissue sample, we employ an avidin pulldown strategy to isolate these proteins. Subsequent liquid chromatography-tandem mass spectrometry (LC-MS/MS) is then employed to decode the intricate details of the subcellular proteome, achieving high-resolution, high-sensitivity, and high-specificity results.
The efficacy of the Microscoop for Scoop Biology is evidenced by its successful employment in the identification of novel proteins integral to various cellular structures, including stress granules, primary cilia, immune synapses, TDP-43 aggregates, and amyloid ß plaques. Each discovery adds a new layer of understanding to the complex puzzle of cellular biology, paving the way for future advancements in diagnostics and therapeutic interventions at the microscopic level.
| Info@syncell.com | |
| 617-631-2746 | |
|
|
200 Dexter Ave, Watertown, MA 02472, USA |
|
|
|